Adeno-Associated Virus Gene Therapy Market Size, Demands, Growth, Forecast & Report 2032 | UnivDatos
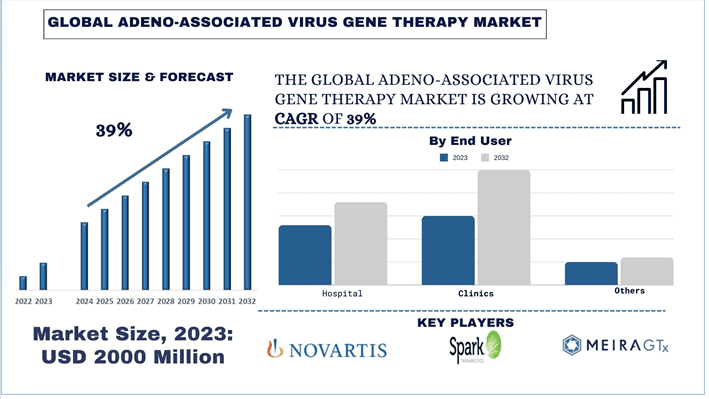
The Adeno-Associated Virus Gene Therapy Market was valued at approximately ~USD 2000 Million in 2023 and is expected to grow at a strong CAGR of around 39% during the forecast period (2024-2032)
Adeno-Associated Virus (AAV) gene therapy has emerged as a groundbreaking approach in the treatment of various genetic and acquired disorders. In the United States, the field has seen remarkable advancements, with numerous clinical trials and FDA approvals highlighting its potential to revolutionize healthcare. This blog explores the current landscape of AAV gene therapy in the U.S., focusing on recent clinical trials and FDA approvals that are shaping the future of medical treatment.
What is AAV Gene Therapy?
AAV gene therapy involves using adeno-associated viruses as vectors to deliver therapeutic genes to patients' cells. These viruses are engineered to be non-pathogenic and have shown a high degree of safety and efficacy in targeting specific cells without integrating into the host genome, which reduces the risk of mutagenesis. This method has shown promise in treating a range of conditions, from rare genetic disorders to more common diseases like heart failure and neurodegenerative diseases.
Recent Clinical Trials
The U.S. has become a hub for cutting-edge AAV gene therapy research, with several companies conducting clinical trials to test the efficacy and safety of their innovative treatments. Here are some noteworthy clinical trials:
Spark Therapeutics - SPK-8011 Spark Therapeutics has been at the forefront of AAV gene therapy, particularly for haemophilia. Their ongoing Phase 1/2 clinical trial for SPK-8011 aims to treat Haemophilia A by delivering a functional copy of the FVIII gene. Initial results have been promising, showing sustained factor VIII activity levels in patients, which could significantly reduce bleeding episodes and the need for regular infusions.
Audentes Therapeutics - AT132 Audentes Therapeutics, a subsidiary of Astellas Pharma, is conducting a Phase 1/2 clinical trial for AT132, aimed at treating X-linked Myotubular Myopathy (XLMTM). This rare neuromuscular disorder affects skeletal muscle function, and early trial results have demonstrated improvements in respiratory and motor functions, offering hope for a condition that previously had limited treatment options.
Biogen - BIIB093 Biogen is exploring AAV gene therapy for neurological disorders, with a focus on delivering a therapeutic gene to treat Amyotrophic Lateral Sclerosis (ALS). Their Phase 1 clinical trial of BIIB093 involves administering the therapy directly to the spinal cord. Early safety data has been encouraging, and further efficacy results are eagerly awaited by the medical community.
Pfizer - PF-06939926 Pfizer's Phase 3 clinical trial for Duchenne Muscular Dystrophy (DMD) with PF-06939926 is one of the most advanced AAV gene therapy studies. This therapy aims to provide a functional version of the dystrophin gene, which is deficient in DMD patients. Preliminary data indicates improved muscle function and strength, potentially transforming the treatment landscape for this debilitating disease.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/adeno-associated-virus-gene-therapy-market?popup=report-enquiry
FDA Approvals
The FDA has played a crucial role in advancing the field of AAV gene therapy by granting approvals and designations that facilitate the development and commercialization of these therapies. Here are some notable FDA approvals:
Luxturna (voretigene neparvovec) Developed by Spark Therapeutics, Luxturna was the first FDA-approved AAV gene therapy for treating inherited retinal disease caused by mutations in the RPE65 gene. Approved in December 2017, Luxturna demonstrated significant improvements in vision for patients with Leber congenital amaurosis and retinitis pigmentosa, marking a milestone in gene therapy.
Zolgensma (onasemnogene abeparvovec-xioi) Zolgensma, developed by AveXis (a Novartis company), received FDA approval in May 2019 for the treatment of spinal muscular atrophy (SMA) in paediatric patients under two years old. This one-time intravenous infusion delivers a copy of the SMN1 gene, addressing the genetic root cause of SMA. Zolgensma's approval underscored the potential of AAV gene therapy to treat severe genetic disorders early in life.
Roctavian (valoctocogene roxaparvovec) BioMarin Pharmaceutical's Roctavian received FDA approval in August 2022 for treating adults with severe Haemophilia A. This AAV-based gene therapy delivers a functional copy of the FVIII gene, providing long-term benefits and reducing the need for frequent factor VIII infusions. Roctavian's approval represents a significant advancement in haemophilia treatment.
Hemgenix (etranacogene dezaparvovec) Approved in November 2022, Hemgenix is another breakthrough for haemophilia, specifically for Haemophilia B. Developed by CSL Behring, this gene therapy introduces a functional copy of the F9 gene, leading to increased production of factor IX. Hemgenix offers the potential for sustained treatment efficacy, reducing or eliminating the need for regular factor IX replacement therapy.
Click here to view the Report Description & TOC: https://univdatos.com/reports/adeno-associated-virus-gene-therapy-market
The Future of AAV Gene Therapy
The success of these clinical trials and FDA approvals underscores the transformative potential of AAV gene therapy. However, challenges remain, including manufacturing scalability, long-term safety, and the need for precise targeting to avoid off-target effects. Researchers and companies are continually working to address these issues through advanced vector engineering, improved delivery methods, and enhanced patient monitoring.
Furthermore, the regulatory landscape is evolving to keep pace with the rapid advancements in gene therapy. The FDA has introduced several initiatives to streamline the approval process for promising therapies, including the Regenerative Medicine Advanced Therapy (RMAT) designation, which provides expedited review and approval pathways for regenerative medicine products.
Conclusion
The U.S. AAV gene therapy market is at the cusp of a revolution, driven by innovative research, successful clinical trials, and supportive regulatory frameworks. The recent approvals of therapies like Luxturna, Zolgensma, Roctavian, and Hemgenix highlight the significant progress made in this field. As more therapies advance through clinical trials and receive FDA approval, AAV gene therapy holds the promise of not only treating but potentially curing a range of genetic and acquired disorders, offering new hope to patients and transforming the future of medicine.
Contact Us:
UnivDatos
Email: contact@univdatos.com
Contact no: +1 978 7330253
Website: www.univdatos.com
Linked In: https://www.linkedin.com/company/univ-datos/


