North America Drug Safety Solutions And Pharmacovigilance Market Opportunities: Growth, Share, Value, Size, and Scope By 2033
"Future of Executive Summary North America Drug Safety Solutions And Pharmacovigilance Market: Size and Share Dynamics
- The North America drug safety solutions and pharmacovigilance market size was valued at USD 2.04 billion in 2025 and is expected to reach USD 5.75 billion by 2033, at a CAGR of 13.85% during the forecast period.
The North America Drug Safety Solutions And Pharmacovigilance Market report offers an analytical assessment of the prime challenges faced by the North America Drug Safety Solutions And Pharmacovigilance Market industry currently and in the coming years, with which market participants can know the problems they may face while operating in this market over a longer period of time. This North America Drug Safety Solutions And Pharmacovigilance Market report has a chapter on the Global North America Drug Safety Solutions And Pharmacovigilance Market and all its associated companies with their profiles, which provides valuable data related to their outlook in terms of finances, product portfolios, investment plans, and marketing and business strategies. By providing trustworthy market research information, this North America Drug Safety Solutions And Pharmacovigilance Market report helps to extend your reach to success in your business.
North America Drug Safety Solutions And Pharmacovigilance Market Research Report provides market forecast information, considering the history of the industry and the future of the industry with respect to what situation it may face and whether it will grow or fail. Inputs of various industry experts, required for the detailed market analysis, have been used very carefully to structure this finest North America Drug Safety Solutions And Pharmacovigilance Market research report. A team of innovative analysts, enthusiastic forecasters, knowledgeable researchers, and experienced industry experts work meticulously, 24/7, to structure this most excellent market report. The research study carried out in this North America Drug Safety Solutions And Pharmacovigilance Market report covers the local and regional as well as the global market.
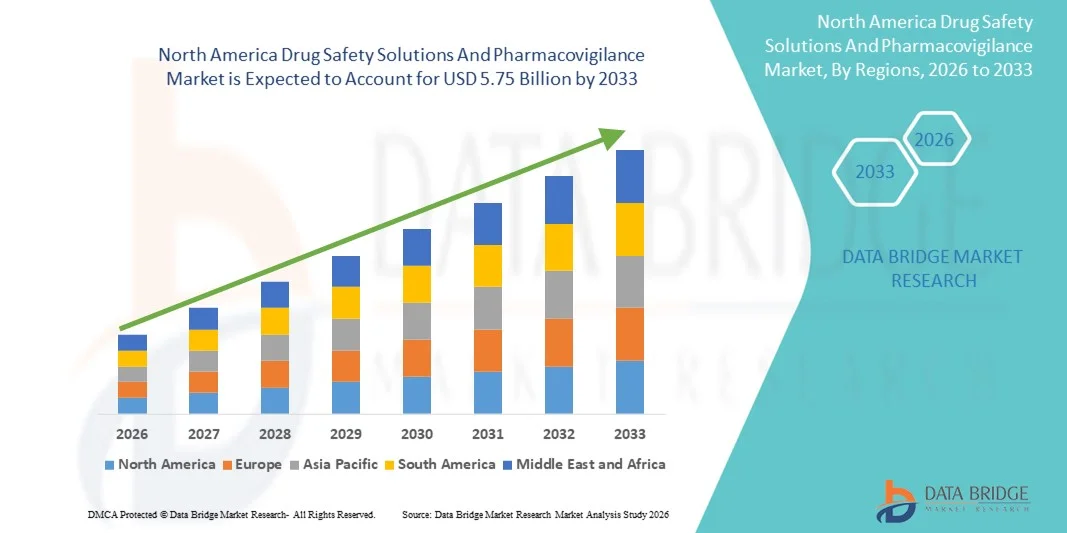
Tap into future trends and opportunities shaping the North America Drug Safety Solutions And Pharmacovigilance Market. Download the complete report:
https://www.databridgemarketresearch.com/reports/north-america-drug-safety-solutions-and-pharmacovigilance-market
North America Drug Safety Solutions And Pharmacovigilance Market Environment
Segments
- The North America Drug Safety Solutions and Pharmacovigilance market can be segmented based on type into adverse event reporting, drug safety audits, issue tracking, fully integrated software, and others. Adverse event reporting holds a significant share in the market as it plays a crucial role in drug safety management by identifying, tracking, and reporting adverse events related to pharmaceutical products. Drug safety audits are also gaining traction as they help in assessing the compliance of drug safety measures with regulatory standards. Issue tracking is essential for monitoring and resolving drug safety issues efficiently. Fully integrated software solutions are increasingly being adopted by pharmaceutical companies for comprehensive drug safety management.
- Based on the delivery mode, the market can be categorized into on-premise solutions and cloud-based solutions. On-premise solutions provide enhanced control and security over data but require higher initial investments. Conversely, cloud-based solutions offer flexibility, scalability, and cost-effectiveness, driving their adoption among small and medium-sized enterprises in the pharmaceutical industry.
- In terms of end-users, the market can be divided into pharmaceutical and biotechnology companies, contract research organizations (CROs), business process outsourcing (BPO) firms, and others. Pharmaceutical and biotechnology companies account for a significant share in the market due to the increasing focus on ensuring drug safety and complying with regulatory requirements. CROs and BPO firms are leveraging drug safety solutions and pharmacovigilance services to streamline their operations and enhance efficiency.
Market Players
- Some of the key players operating in the North America Drug Safety Solutions and Pharmacovigilance market include Oracle Corporation, IQVIA, SAS Institute Inc., Sparta Systems, Inc., DXC Technology Company, EXTEDO GmbH, LINICAL Accelovance, Online Business Applications, Inc., Sarjen Systems Pvt. Ltd., and ArisGlobal. These companies are focusing on innovating their product offerings, expanding their geographical presence, and forging strategic partnerships to gain a competitive edge in the market.
- Additionally, regulatory authorities such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are taking stringent measures to ensure drug safety and pharmacovigilance, further propelling the demand for advanced solutions in the market. The increasing prevalence of adverse drug reactions and the rising number of clinical trials are also driving the growth of the market in North America.
In addition to the segmentation already discussed, further analysis of the North America Drug Safety Solutions and Pharmacovigilance market reveals emerging trends and factors shaping the industry landscape. One noteworthy trend is the increasing adoption of artificial intelligence (AI) and machine learning (ML) technologies in drug safety solutions. These advanced technologies enable the automation of adverse event detection, signal detection, and trend analysis, thus enhancing the efficiency and accuracy of pharmacovigilance processes. Pharmaceutical companies are leveraging AI and ML to sift through vast amounts of data and identify potential safety issues promptly.
Another significant factor impacting the market is the growing emphasis on real-world evidence (RWE) in pharmacovigilance practices. Real-world data derived from sources such as electronic health records, patient registries, and wearables provide valuable insights into drug safety profiles and long-term outcomes. Integrating RWE into pharmacovigilance strategies allows for a more comprehensive and proactive approach to monitoring drug safety beyond clinical trial settings. This shift towards real-world evidence is reshaping the traditional pharmacovigilance landscape and driving the demand for sophisticated solutions capable of handling diverse data sources.
Moreover, the increasing globalization of clinical trials and drug development activities is influencing the dynamics of the North America Drug Safety Solutions and Pharmacovigilance market. Collaborations between multinational pharmaceutical companies, CROs, and regulatory bodies from different regions necessitate standardized pharmacovigilance practices and data sharing mechanisms. As a result, there is a growing need for interoperable and scalable drug safety solutions that can ensure compliance with varying regulatory requirements across different geographies while maintaining data integrity and patient confidentiality.
Furthermore, the evolving regulatory landscape, including the implementation of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines and Good Pharmacovigilance Practices (GVP), is driving the adoption of sophisticated pharmacovigilance solutions in North America. Companies are investing in systems that can facilitate compliance with regulatory obligations, ensure timely reporting of adverse events, and support proactive risk management strategies. The convergence of regulatory pressures, technological advancements, and global collaborations is fueling the demand for comprehensive drug safety solutions that can cater to the evolving needs of the pharmaceutical industry.
In conclusion, the North America Drug Safety Solutions and Pharmacovigilance market are undergoing significant transformations driven by trends such as AI adoption, real-world evidence integration, globalization of clinical trials, and regulatory developments. Market players need to stay abreast of these trends and align their product offerings with the evolving requirements of the industry to maintain a competitive edge. By embracing innovation, technology, and regulatory compliance, companies can capitalize on the growing demand for advanced drug safety solutions and contribute to enhancing patient safety and healthcare outcomes.The North America Drug Safety Solutions and Pharmacovigilance market is witnessing a paradigm shift driven by emerging trends and evolving factors that are reshaping the industry landscape. One of the key trends making a significant impact is the increasing adoption of artificial intelligence (AI) and machine learning (ML) technologies in drug safety solutions. These advanced technologies are revolutionizing pharmacovigilance processes by automating adverse event detection, signal identification, and trend analysis, thereby enhancing operational efficiency and accuracy. Pharmaceutical companies are leveraging AI and ML algorithms to sift through vast datasets swiftly, enabling the timely identification of potential safety issues and proactive risk management.
Another crucial factor shaping the market is the rise of real-world evidence (RWE) in pharmacovigilance practices. Real-world data derived from diverse sources such as electronic health records and patient registries offer valuable insights into drug safety profiles and long-term treatment outcomes. The integration of RWE into pharmacovigilance strategies allows for a holistic approach to monitoring drug safety beyond the confines of clinical trials, enabling stakeholders to proactively identify and address emerging safety concerns. This shift towards RWE is driving the demand for advanced pharmacovigilance solutions capable of handling complex and varied data sources effectively.
Furthermore, the globalization of clinical trials and drug development activities is exerting a significant influence on the dynamics of the North America Drug Safety Solutions and Pharmacovigilance market. Increasing collaborations between multinational pharmaceutical companies, contract research organizations, and regulatory bodies from different regions necessitate standardized pharmacovigilance practices and data sharing mechanisms. This trend underscores the need for interoperable and scalable drug safety solutions that can ensure regulatory compliance across diverse geographies while upholding data integrity and patient confidentiality. As the industry becomes more interconnected and globalized, the demand for robust pharmacovigilance solutions that can adapt to varying regulatory landscapes is expected to rise.
Moreover, the evolving regulatory environment, including the implementation of international guidelines such as ICH and GVP, is compelling companies to invest in sophisticated pharmacovigilance solutions to meet compliance requirements. Organizations are focusing on enhancing reporting capabilities, proactive risk management, and ensuring timely adverse event notifications to meet regulatory obligations. The convergence of regulatory pressures, technological advancements, and increased global collaborations underscores the importance of comprehensive drug safety solutions that can address the evolving needs of the pharmaceutical industry in North America.
In conclusion, the North America Drug Safety Solutions and Pharmacovigilance market is undergoing a transformative phase driven by trends like AI adoption, RWE integration, globalization of clinical trials, and regulatory developments. Market players need to align their strategies and product offerings with these trends to stay competitive and meet the changing demands of the industry. By leveraging innovation, technology, and regulatory compliance, companies can capitalize on the growing demand for advanced drug safety solutions, ultimately contributing to improved patient safety and healthcare outcomes in the region.
Evaluate the company’s influence on the market
https://www.databridgemarketresearch.com/reports/north-america-drug-safety-solutions-and-pharmacovigilance-market/companies
Nucleus is Data Bridge Market Research’s cutting-edge, cloud-based market intelligence platform that empowers organizations to make faster, smarter, data-driven decisions. Designed for strategic thinkers, researchers, and innovators, Nucleus transforms complex macroeconomic indicators, industry-specific trends, and competitive data into actionable insights through dynamic dashboards and real-time analytics. With capabilities spanning market access intelligence, competitive benchmarking, epidemiological analytics, global trade insights, and cross-sector strategy modeling, the platform unifies diverse datasets to help businesses identify opportunities, assess risks, and drive growth across regions and industries. Built on a powerful neural analytics engine, Nucleus bridges the gap between raw data and strategic execution, enabling users to visualize emerging trends, benchmark performance, and make informed decisions with confidence.
Get More Detail: https://www.databridgemarketresearch.com/nucleus/north-america-drug-safety-solutions-and-pharmacovigilance-market
Forecast, Segmentation & Competitive Analysis Questions for North America Drug Safety Solutions And Pharmacovigilance Market
- What’s the estimated market worth of North America Drug Safety Solutions And Pharmacovigilance Market globally?
- How is North America Drug Safety Solutions And Pharmacovigilance Market growth distributed across regions?
- Which segment generates the highest revenue for North America Drug Safety Solutions And Pharmacovigilance Market?
- What companies are discussed in the strategic landscape for North America Drug Safety Solutions And Pharmacovigilance Market?
- Which countries are experiencing rapid adoption in North America Drug Safety Solutions And Pharmacovigilance Market?
- Who are the globally recognized competitors in North America Drug Safety Solutions And Pharmacovigilance Market?
Browse More Reports:
Global Ventilated Seats Market
Global Veterinary Oncology Market
Global Video Telemedicine Market
Global Viral Conjunctivitis Market
Global Virtual Reality Neuropsychological Therapy Market
Global Visual Cloud Market
Global Vitreoretinal Surgery Devices Market
Global Voice Assistant Application Market
Global Voice Over Internet Protocol Market
Global Von Willebrand Disease Treatment Market
Global Washable Markers Market
Global Waterborne Coatings Market
Global Waterproof Socks Market
Global Weatherization Services Market
Global Wegener’s Granulomatosis Treatment Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"


